The treatment of a huge number of diseases, including cancers, is tightly related to our capacity of understanding and harnessing the development of cell populations. Cell characterization and cell sorting are thus tremendous challenges for therapies of the future. In this framework, Advanced Therapy Medical Products (ATMPs) recently emerged in order to provide new therapy solutions for patients in therapeutic impasse or for new therapies. These ATMPs rely on the use of “drug cells” exhibiting new physiological functions, biological characteristics or reconstitution properties directly inspired from natural processes occurring in the human organism. However, developing these drug cells necessitates extracting a few lymphocytes from a blood sample.
The Micro and Nano Robotics team conducts research works to develop lab on chip for highly selective cell sorting based on robotic approaches. Its goal is to propose the next generation of automated cell sorters in the framework the development of Advanced Therapy Medical Products.
To increase the selectivity of classical cell sorters we propose to use a fluidic chip actuated by DEP forces and controlled by closed loop control. This necessitates two main elements: a model of the chip that can compute in real time the position of the cells in the chip depending on the voltage applied on the electrodes, and sensors to monitor in real time the position of the cells in the channels.
Dielectrophoretic actuation
The actuation inside the fluidic chip is performed by dielectrophoreis. Dielectrophoresis force (DEP force) is the force induced on a polarizable particle suspended in a non-uniform electric field. Based on the analysis of the dielectrophoretic behavior of particles, it has been demonstrated that DEP is an effective means for micro-manipulation, deposition and micro assembly.
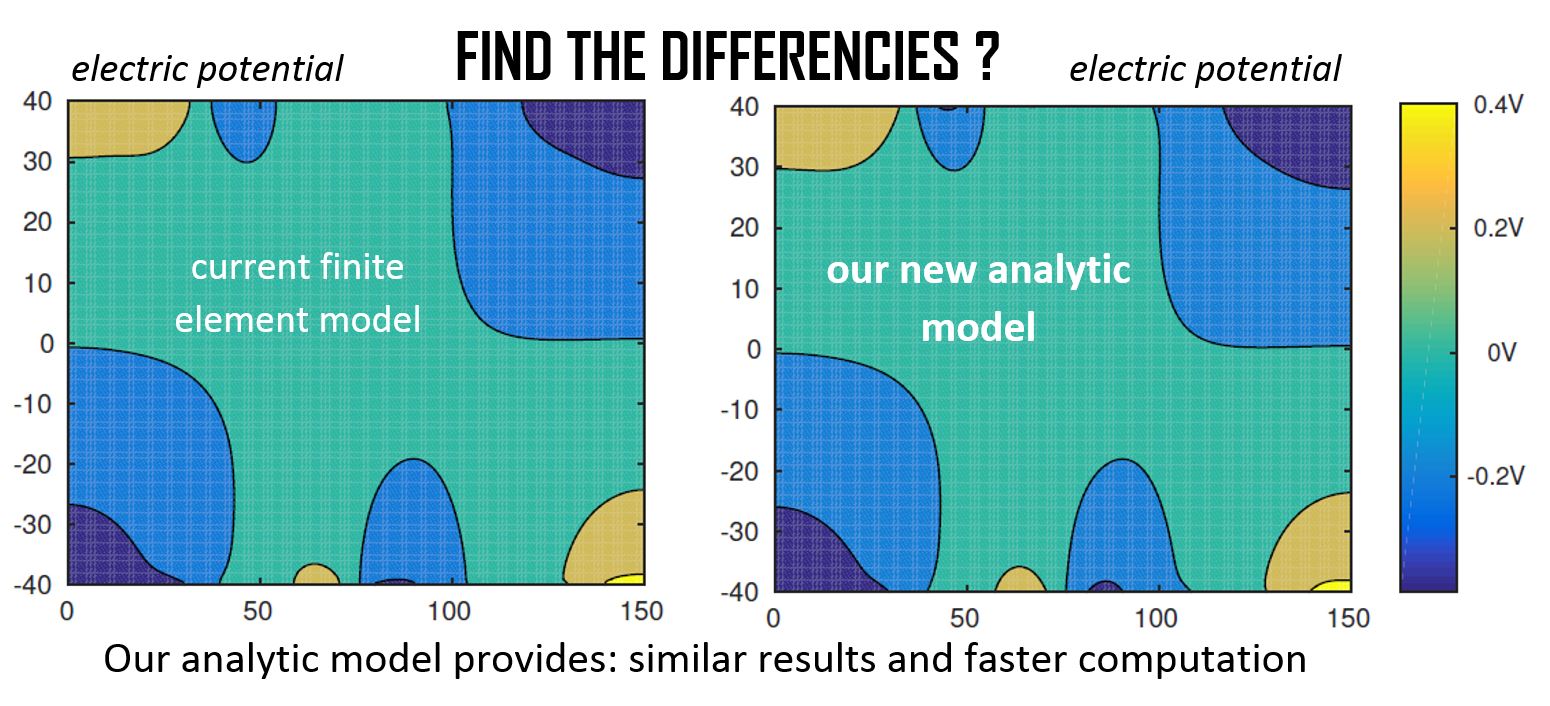
Currently, the numerical models of dielectrophoretic force are too complex to be used in real-time closed-loop control. The aim of this research is to propose a new type of models usable in this framework. We propose an analytical model of the electric field based on Fourier series to compute the dielectrophoretic force produced by parallel electrode arrays. Indeed, this method provides an analytical expression of the electric potential which decouples the geometrical factors (parameters of the system), the voltages applied on electrodes (input of the system), and the position of the cells (output of the system). Considering the Newton laws on each cell, it enables to generate easily a dynamic model of the cell positions (output) function of the voltages on electrodes (input). This dynamic model of the system is required to design the future closed-loop control law. The model developed enables to compute the dielectrophoretic force applied to a cell by an electrode array in a few tenths of milliseconds. This model will be used in future works to perform closed loop control: on-going works.
Position estimation using impedancemetry
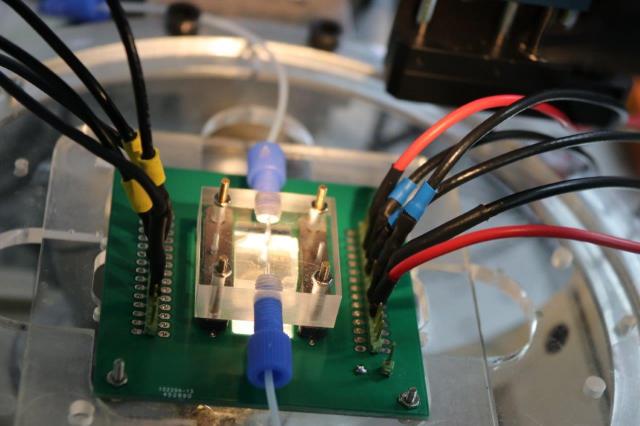
To perform closed loop control based on this model a second issue must be solved: the detection in real time of the position of the cells inside the microfluidic chip.Due to the high number of cells inside the chip that must be monitored at the same time, classical vision-based sensors are not suitable. We propose an alternative based on impedance measurement. The position of the cells is obtained from the variation of impedance measured between two electrodes. This technique presents several advantages: the sensor is integrated into the chip, the measurement electrodes are compatible with the fabrication process of actuation electrodes for dielectrophoresis, the sampling time of the sensor is high and the position of the cells can be obtained in real time. The issue of real-time detection in a noisy environment is solved by using an Extended Kalman Filter. As a first proof of concept we present an experimental validation on a 1D case to determine the longitudinal position of 8.7 µm diameter beads in a channel. Experimental validation on a 2D position estimation is currently investigated. This work is done in collaboration with EPFL.
Contact
Aude Bolopion
Involved people
Current people: Hugo Daguerre, Belal Ahmad, Vladimir Gauthier
Former people: Benoit Brazey, Tomas Michalek (AA4CC, Czech Republic)