Context
Each year, several millions of red cell concentrates are transfused worldwide. For every single blood bag, a pre-transfusion test is performed. It always consists in verifying the patient’s identity and performing a compatibility test. Different test procedures are observed depending on the country. It can be an ABO compatibility test performed manually at the patient’s bedside or automatically in a laboratory. It can also be what is called “crossmatch”, performed either manually or automatically, but always in a biology laboratory. However, despite these tests, transfusion accidents still occur. In order to avoid them, pre-transfusion test should ideally be performed automatically at the patient’s bedside.
The SmarTTransfuser program aims at developing such automated device in a mobile form and easy to use by non-trained people. The program is managed by the Clinical Investigation Center of Besançon University Hospital in collaboration with the French Blood Agency and the FEMTO-ST Institute.
SmarTTransfuser program is composed of several distinct but complementary projects:
- the SmarTTransfuser project aiming at replacing the manual compatibility test at the patient’s bedside
- the ABORDAGE project aiming at adding the detection of the RH1 to SmarTTransfuser
- the X-ult project aiming at performing a simplified crossmatch at the patient’s bedside.
Therefore, the whole program will offer solutions to improve the transfusion safety, for the patient but also for the medical staff, to provide an objective measurement of the “donor-acceptor” compatibility and to automate the ultimate pre-transfusion procedures at the patient’s bedside.
SmarTTransfuser project
Biochips containing the same reaction zone as the ones present on the current cardbox test are placed in disposable cartridges. The mobile medical device is placed in the transfusion line and ABO compatibility test is performed automatically. Optical absorption reaction reading allows an objective interpretation of the test. SmarTTransfuser project will help re-enforcing the transfusion safety for both patient and medical staff.
Up to now, we have validated a biosensor which allows detecting ABO compatibility by means of biochips capturing red cells according to their group. Grafting of anti-A and anti-B antibodies on the biochip surface (figure 1) as well as efficiency of red cell capture (figure 2) is confirmed. Specificity of the capture as well as performance of the optical absorption reading in a laboratory model and in a pre-industrial prototype is also confirmed (figure 3).
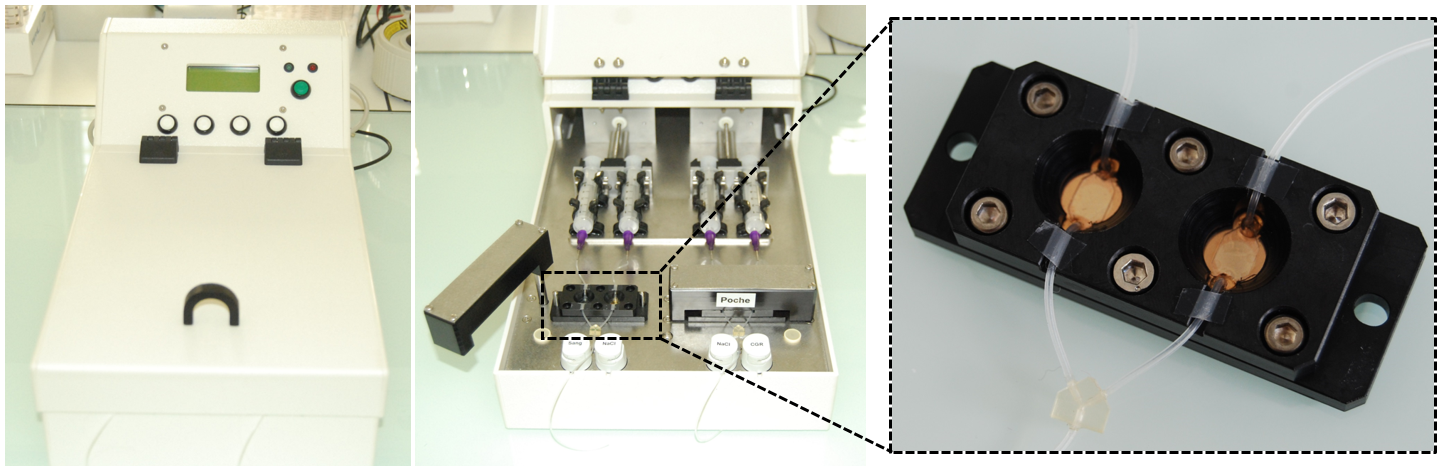 |
| Figure 1 : Views of the SmarTTransfuser prototype |
This project has produced 2 patents, obtained the prize of the best poster at the Biosensing Technology conference in 2012. It has also been presented in many international conferences or workshop and obtained an INSERM Grand Est award in the scientific picture category.
The prototype has been clinically validated by the Clinical Investigation Center of Besançon University Hospital in collaboration with the haemo-vigilance unit.
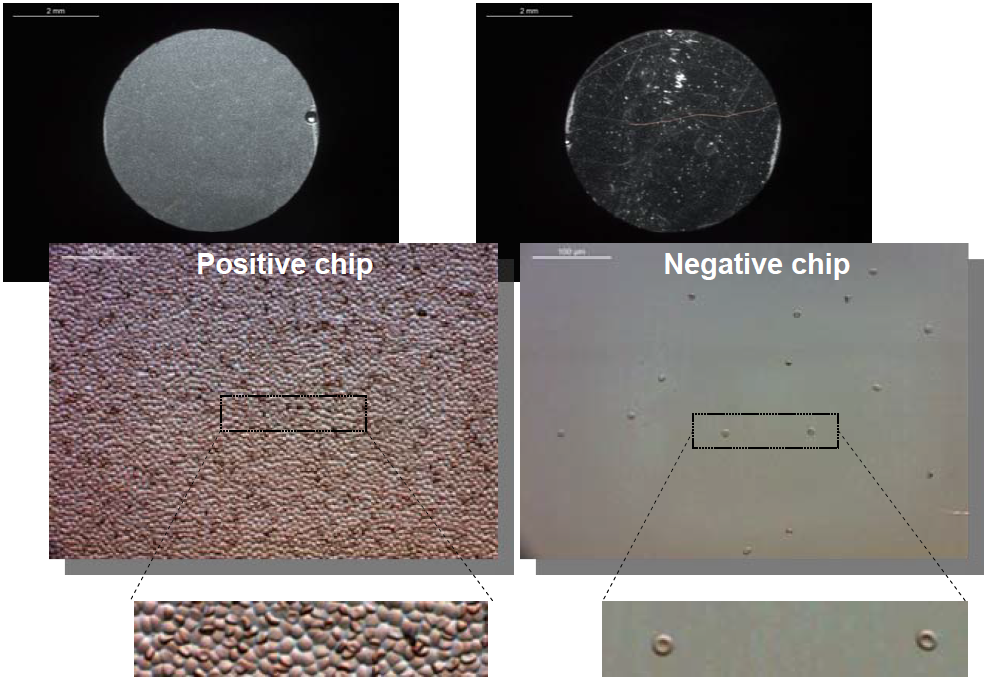 |
| Figure 2 : Left: positive biochip; the surface is entirely covered by red cell. Right: negative biochip; only a very few red cells can be seen. |
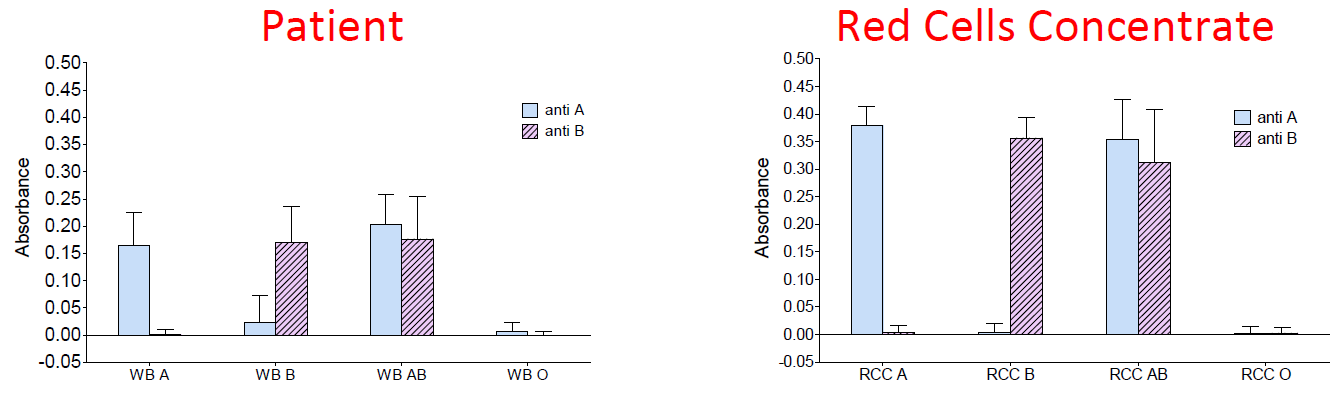 |
| Figure 3 : optical absorbance of pairs of biochips (anti-A and anti-B) for different blood groups. Left: whole blood. Right: red cell concentrate. |
ABORDAGE Project
Following SmarTTransfuser, the ABORDAGE project started in 2013. It aims at adding a new functionality by detecting the RH1 rhesus compatibility. First tests showed an efficient capture of the red cell on biochips grafted with anti-D antibodies even with low hematocrit levels.
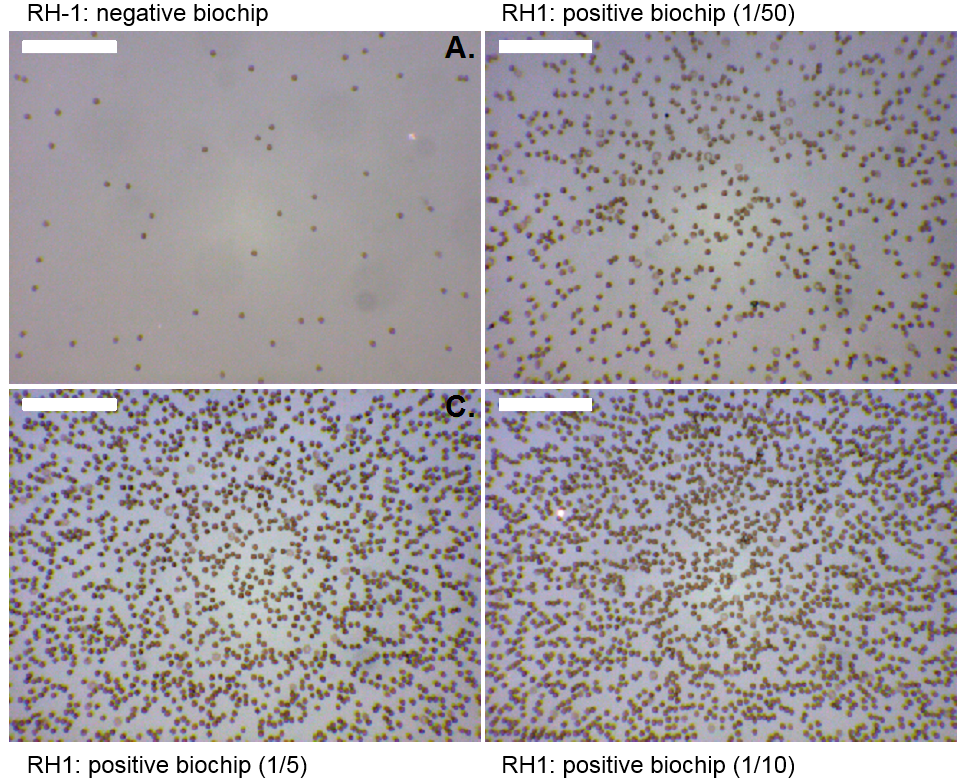 |
| Figure 4 : Selectively captured red cell on the surface of the biochips. Top left: negative biochip. Other pictures with varying red cell concentrations. Current work consists in testing the optical absorbance reading. This part of the web site should be up-dated shortly. |
X-ult Project
This project aims at reproducing, at the patient’s bedside, the crossmatch test performed in many countries in a biology laboratory. It has been funded by the National French Blood Agency recently.
.
Publications related to this programme
- L. Pazart, B. Wacogne, C. Pieralli, W. Boireau et P. Morel, "Dispositif de prélèvement de liquide corporel et procédé de mise en oeuvre", publication brevet français enregistrée le 9 Novembre 2009, N° 0905329.). "DEVICE FOR TAKING A SAMPLE OF A BODY FLUID AND METHOD FOR IMPLEMENTING SAME". Extension PCT 12 Mai 2011, N° WO2011055029.
- L. Pazart, B. Wacogne, C. Pieralli, W. Boireau et P. Morel, "Système de perfusion sécurisé et procédé de mise en oeuvre", publication brevet français enregistrée le 5 Novembre 2009, N° 0905330. "SECURE PERFUSION SYSTEM". Extension PCT 12 Mai 2011, N° WO2011055031.
- "On-arrays capture of red blood cells in critical transfusional cases", W. Boireau, C. Elie-Caille, A. Rouleau, J.S. Guerrini, B. Wacogne C. Pieralli and L. Pazart, conference invitée, World Congress on Biotechnology, 21-23 March 2011, Hyderabad, India.
- "SmartTransfuser: a biochip system for the final ABO compatibility test", K. Charrière, J.S. Guerrini-Chapuis, B. Wacogne, A. Rouleau, C. Elie-Caille, C. Pieralli, L. Pazart, P. Morel and W. Boireau, Conférence Biodevices 2012, Vilamoura, Portugal, In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 257-262. DOI: 10.5220/0003852402570262.
- “On-arrays capture of red blood cells in critical transfusional cases”, W. Boireau, K. Charrière, C. Elie-Caille, A. Rouleau, J.S. Guerrini, B. Wacogne, C. Pieralli, and L. Pazart, Journée de l’IFR 133, 27 Mai 2011, Besançon, France.
- "La recherche translationnelle au service de la sécurité transfusionnelle. Dispositif automatisé de contrôle de la compatibilité sanguine: SmarTTransfuser", K. Charrière, O. Gaiffe, A. Rouleau, W. Boireau, P. Morel, L. Pazart and B. Wacogne, Journée de la SFR FED 4234, 2012, Besançon, France.
- "The required steps in the development of a medical device: an example in the frame of the transfusion safety", K. Charriere, O. Gaiffe, L. Pazart, W. Boireau, C. Pieralli and B. Wacogne, RGB 2013, 1st Russian German conference, 23-26 October 2013, Hanover, Germany.
- "Nano-bio-engineering on µarrays for the capture of red blood cells in critical transfusional cases", W. Boireau, C. Elie-Caille, A. Rouleau, J.S. Guerrini-Chapuis, B. Wacogne, C. Pieralli and L. Pazart, poster, 11th World Congress on Biosensors, May 26-28 2010, Glasgow, Scotland, 2010.
- "SmartTransfuser: a lab-on-chip system for enhancing transfusion security", K. Charrière, J.S. Guerrini-Chapuis, B. Wacogne, C. Elie-Caille, C. Pieralli, L. Pazart, P. Morel and W. Boireau, Poster, 2nd International Conference on Bio-sensing Technology, 10-12 October 2011, Amsterdam, The Nederlands. Prix du meilleur poster.
- Prix du meilleur poster au congrès "SmartTransfuser: a lab-on-chip system for enhancing transfusion security", K. Charrière, J.S. Guerrini-Chapuis, B. Wacogne, C. Elie-Caille, C. Pieralli, L. Pazart, P. Morel and W. Boireau, Poster, 2nd International Conference on Bio-sensing Technology, 10-12 October 2011, Amsterdam, The Nederlands.
- "La recherche translationnelle au service de la sécurité transfusionnelle. Dispositif automatisé de contrôle de la compatibilité sanguine: SmarTTransfuser", K. Charrière, O. Gaiffe, A. Rouleau, W. Boireau, P. Morel, L. Pazart and B. Wacogne, Journée de la SFR FED 4234, 2012, Besançon, France.
- "Biochip technology applied to an automated ABO compatibility test at the patient bedside", Charrière K., Rouleau A., Gaiffe O., Fertey J., Morel P., Bourcier V., Pieralli C., Boireau W., Pazart L., Wacogne B., Journée de la SFR FRD, 23 Mai 2014, Besançon, France.
- "Optical detection of red blood cells immunocaptured by biochips in a mobile device called SmarTTransfuser", K. Charrière, O.Gaiffe, C. Pieralli, L. Pazart, P. Morel, W. Boireau, and B. Wacogne, Conference, 5th International Conference on Photonics, 2-4 September 2014, Kuala Lumpur, Malaysia. IEEE Proceedings ICP, pp. 153-155, DOI: 10.1109/ICP.2014.7002340.
- Biochip technology applied to an automated ABO compatibility test at the patient bedside", K. Charrière, A. Rouleau, O. Gaiffe, J. Fertey, P. Morel, V. Bourcier, C. Pieralli, W. Boireau, L. Pazart, B. Wacogne, Sensors and Actuators B, Vol 208, pp. 67-74, 2015. http://dx.doi.org/10.1016/j.snb.2014.10.123
- "An automated medical device for ultimate ABO compatibility test at the patient’s bedside - Towards the automation of point-of-care transfusion safety", Karine Charrière, Alain Rouleau, Olivier Gaiffe, Pascal Morel, Véronique Bourcier, Christian Pieralli, Wilfrid Boireau, Lionel Pazart and Bruno Wacogne, Conference Biodevices 2015, 12 - 15 january, Lisbon, Portugal, In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 58-67. DOI: 10.5220/0005248700580067.
- "Immuno-opto-fluidic biochip for point-of-care transfusion safety", Karine Charrière, Alain Rouleau, Pascal Morel, Véronique Bourcier, Christian Pieralli, Wilfrid Boireau, Lionel Pazart and Bruno Wacogne, International Conference on Small Science, ICSS 2015, 4-7 november 2015, Phuket, Thailand.
- "Une recherche pluri-science et transdisciplinaire pour le développement de dispositifs intelligents en santé Biom@x, CLIP et SmartTransfuser", Bruno Wacogne, Wilfrid Boireau, Diagnostic and Medical Device B4B-Connection, 23-24 mars 2016, Besançon.
- "Integrated device based on red cell capture on protein biochips for automated ABO compatibility control at the patient's bedside", Karine Charrière, Alain Rouleau, Pascal Morel, Véronique Bourcier, Christian Pieralli, Wilfrid Boireau, Lionel Pazart and Bruno Wacogne, Conference, Bio-sensing technology, 10-13 Mai 2015, Lisbonne, Portugal.
- “Optical detection of red blood cells captured on biochips for RH1 compatibility control at the patient’s bedside”, K. Charrière, A. Guitton, C. Tissot, A. Rouleau, P. Morel, V. Bourcier, W. Boireau, L. Pazart, B. Wacogne, Conférence Biodevices 2016, 21 - 23 february, Rome, Italy.

